OVERVIEW
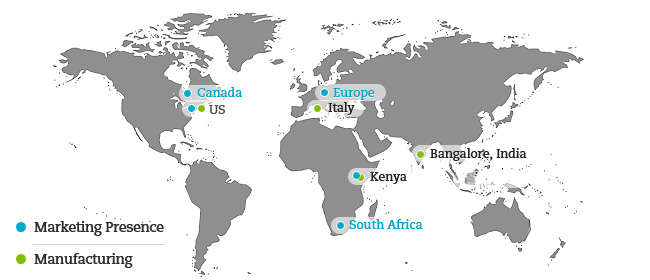
Our domain strategy of developing a comprehensive product portfolio in complex therapeutic segments has attracted marquee partnerships in Regulated markets such as US, Europe, Australia, Canada and S. Africa.
Our efforts on automation in the areas of manufacturing and laboratory has resulted in more activities coming in the scope of automated environment for us, and thus complementing our initiatives for greater compliance. We are now poised for leveraging these actions for more extensive actions for maximizing the benefits and thus creating a more rewarding and conducive environment for Quality and Compliance.
We have also increased our Data and Compliance governance mechanisms and used them effectively for continuous improvements. The risk management process has been enhanced by bringing newer areas or domains into the risk management process, so to address the concerns where needed systematically.
Manufacturing facilities
 Facility Location: Bangalore,
Karnataka
Facility Location: Bangalore,
Karnataka
- Tablets
- Hard Gelatin Capsules
- Soft Gelatin Capsules
- Sachets
- Liquids [Oral Liquids)
- Topicals [Ointment & Cream] and Powders
- US-FDA
- MHRA
- ANVISA
- TGA
- WHO
 Facility Location: Puducherry, India
Facility Location: Puducherry, India
- Tablets
- Hard Gelatin Capsules
- Sachets
- US-FDA
- MHRA
- ANVISA
- Health Canada
- WHO
 Facility Location: Chennai, India
Facility Location: Chennai, India
- Tablets
- Hard Gelatin Capsules
- US-FDA
 Facility Location: Milan, Italy
Facility Location: Milan, Italy
- Semi solids, Ointments & creams, Oral Liquids
- EUGMP
 Facility Location: Chestnut Ridge, New York
Facility Location: Chestnut Ridge, New York
- Oral solids including control substances
- Modified Release products
- Liquids
- Nasal sprays
- Gels
- USFDA
FACTS
- High-end manufacturing facilities in India, Italy, US and Singapore approved by all the leading global regulatory authorities
- Strong R&D capabilities
with a focus on development of IP-led, high-value complex generics - Proficient Regulatory framework capable of
developing and filing
products in major regulated markets - Capability to manufacture varied dosage formats
including combi-packs, bi-layered tablets, sachets,
soft and hard-gel caps - Cutting edge modified-release technologies like
sustained release and delayed release
in various oral dosage forms